Preparation
and Properties of Small Nanoparticles
for Skin and Hair Care
LINK TO OUR
PRINCIPALS: Mibelle AG
Cosmetics, Switzerland
Keywords:
Nanoparticles, Liposomes, UV Protection, Encapsulation,
Vitamins, Submicron Emulsions
|
Summary
Introduction
What Are Nanoparticles?
Preparation and Characterization of Nanoparticles
Unique Properties of Ultra Small Nanoparticles
Stability of Nanoparticles
Activity of Nanoparticles
Small Positively Charged
Nanoparticles for Hair Care
Conclusions
References
Summary
Nanoparticles are small lipid vesicles formed by a monolayer of
phospholipids. Whereas liposomes are typical carriers for
hydrophilic substances, nanoparticles are the ideal delivery
system to transport and protect lipophilic agents.
In our laboratory, we have
developed a method to prepare very small nanoparticles
encapsulating different agents of cosmetic and pharmaceutical
interest (Tretinoin, Retinol, Vitamin E Acetate, UV-Filters,
Fragrance). The technique of high pressure homogenization at 1200
bar using a microfluidizer yields a 100% encapsulation of the oil
in defined vesicles.
The vesicle size has a
great influence on the optical appearance of the nanoparticle
dispersion. Preparations of particles with diameters of less than
60 nm are transparent dispersions of oil in water. These small
nanoparticles show unique additional physical properties and
offer new application possibilities.
Our data show that
nanoparticles are very stable and have a high affinity to the
stratum corneum. Therefore, an enhanced bioavailability of the
encapsulated material to the skin is achieved.
We have also developed a
nanoparticle delivery system to target the vesicles to hair. For
that purpose, we have dotted the nanoparticle shell with cationic
molecules thus producing a positively charged surface. Our
experiments show that positively charged nanoparticles loaded
with UV-filters have an almost one hundred fold higher affinity
to hair than negatively charged particles.
Introduction
Lipid
vesicles were first described by Dr. Alec Bangham in 1965 [ 1 ].
He had observed that handshaken phospholipid dispersions in water
form multilamellar spherical structures. These vesicles, soon
named liposomes, consist of an aqueous cavity encapsulated by one
(Figure 1) or more lipid bilayer membranes. Since these early
investigations, more than 20 years have past till the first
cosmetic products containing liposomes appeared on the market (NIOSOMES
from Lanc?me and CAPTURE from Dior, 1986). However, only three
years later, more than one hundred different liposomal
formulations could be found.
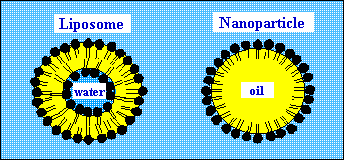
Figure 1.
Comparison of the structure of liposomes and nanoparticles formed
by soy phospholipids.
Liposomes are used to carry and protect hydrophilic agents. Water
soluble agents are enclosed into liposomes if they are present
during the preparation. However, some part of the material always
remains in the outer phase. In addition to water soluble
substances, also amphiphilic and lipophilic substances can be
loaded into liposomes to some extent. Amphiphilic molecules stick
to the membrane whereas lipophilic substances can be incorporated
into the hydrophobic part of the bilayer. Usually such molecules
have a negative influence on the stability of the liposomes.
In contrast to liposomes,
nanoparticles are the ideal carrier system to transport and
protect lipophilic agents.
What
Are Nanoparticles?
Nanoparticles are small lipid vesicles in the range of nanometers.
The best way to characterize them is to compare them with
liposomes and emulsions. Liposomes and nanoparticles are of
comparable size. Both occur in the range from 20 to 1000 nm in
diameter. Whereas liposomes are composed of one or more bilayer
membranes, nanoparticles are formed by a single layered shell (Figure
1). Liposomes are filled with water and therefore are typical
carriers for hydrophilic substances. On the other side,
nanoparticles are filled with oil and lend themselves ideally as
carriers for lipophilic agents.
Nanoparticles can also be
described as a submicron emulsion of oil in water stabilized by a
natural emulsifier. These emulsions are well accepted and used as
delivery system for parenteral drug administration [ 2, 3 ].
Preparation
and Characterization of Nanoparticles
High pressure homogenization using a microfluidizer is a
sophisticated technology to prepare lipid vesicles such as
liposomes and nanoparticles [ 4 ]. The method is easy to scale up
and yields reproducible results. The homogenizer has a specially
designed interaction chamber. In this chamber, the stream of the
premixed components is first divided and then combined again at a
particular angle. At this point, high shear and cavitation forces
form the lipid vesicles at a pressure of up to 1200 bar.
The technique of high
pressure homogenization yields in a 100% encapsulation of
dispersed oil into the vesicles.
Table 1. Correlation of
particle size with concentrations of lecithin and oil
| Lecithin |
Particle Size |
Oil Core |
| 2.5% |
100
nm |
18% |
| 2.5% |
200 nm |
42% |
| 4% |
50
nm |
10% |
| 6% |
40 nm |
10% |
Usually, multiple cycles
through the interaction chamber are necessary to obtain a
homogenous product. The mean droplet size and the size
distribution are the main parameters to characterize nanoparticle
preparations. They can be determined by photon correlation
spectroscopy or by means of electron microscopy of samples
prepared by freeze fracture.
The core of the particles
can contain a wide variety of different cosmetic oils (triglycerides,
jojoba oil, borage oil, wheat germ oil, macadamia nut oil) and
lipophilic agents (vitamin A palmitate, vitamin E acetate,
retinol, tretinoin, UV filters, fragrances). The chemical
stability of these ingredients (against oxidation) can be
enhanced by their encapsulation into nanoparticles [ 5 ].
Nanoparticle preparations
can contain up to 40% of oil. The vesicle size is influenced by
many parameters. Most important are homogenization pressure,
concentration and type of lecithin, concentration and type of oil
and the solvent concentration in the water phase [ 6 ]. Very
small particles can only be achieved at a high ratio of
phospholipid to oil. Table 1 correlates the phospholipid
concentrations of nanoparticle preparations and the encapsulated
oil volumes depending on the size of the vesicles. Preparations
consisting of high concentrations of phospholipids and oil are
very viscous and unstable and therefore not suitable as cosmetic
raw materials.
Unique
Properties of Ultra Small Nanoparticles
The vesicle size has a
great influence on the optical appearance of the nanoparticle
dispersions. Preparations of particles with diameters of 200 nm
or more are white even in diluted dispersions. Preparations
containing particles of 100 nm appear opaque. A further reduction
of the particle size to below 60 nm results in clear transparent
dispersions of oil in water. These preparations offer new
application possibilities in hair care preparations or
transparent hydrogel formulations. In addition, a very high
bioavailability of the encapsulated material to skin and hair is
obtained.
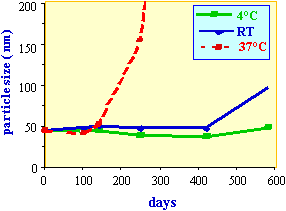
Figure 2.
Stability of a nanoparticle preparation containing 3% vitamine E
acetate and 1% vitamine A palmitate at different temperatures.
As a result of the very small particle size of transparent
preparations, we observed a retarded crystallization of molten
lipids. A nanoparticle dispersion prepared from molten
hydrogenated peanut oil (melting point 35°C) remains a liquid
submicron oil-in-water emulsion (determined by differential
scanning calorimetry) even after the preparation was stored for
several weeks at 4°C. As with the phenomenon of the supercooled
melt, we observed the presence of supersaturated solution of an
UV-filter encapsulated in nanoparticles.
Stability
of Nanoparticles
Nanoparticles are very stable dispersions of oil in water. These
emulsions are stabilized by a negative zeta potential which
prevents droplet coalescence upon random collisions of particles.
The instability of nanoparticles is measured as an increase in
particle size determined by photon correlation spectroscopy. At
high temperature and high particle concentration, the vesicles
start to fuse. An increase of the mean particle size can then be
measured. Figure 2 shows the stability of a nanoparticle
preparation at different temperatures.
The overall negative
charge (zetapotential - 30 mV) on the surface of our particles
results in repulsion forces stabilizing the preparation. The
strength of these forces is strongly reduced when ions are
present. Even low concentrations of salt (50 mM) result in a
quick increase of particle size. Ions and positively charged
polymers must therefore be avoided in cosmetic preparations
containing nanoparticles.
Activity
of Nanoparticles
The most important property of lipid vesicles based on
phospholipids is their affinity to the stratum corneum. A large
number of investigations provide clear evidence that vesicles,
such as liposomes, exert a pronounced influence on the epidermis
[ 7, 8, 9 ]. In an review [ 10 ], Mezei summarizes clinical
investigations which clearly demonstrate that topical
applications of drugs, such as corticosteroids, antifungals,
local anesthetics and retinoids, encapsulated in liposomes result
in increased concentrations of the agents in the epidermis and
dermis compared to conventional formulations. On the other hand,
the systemic concentrations of these drugs (plasma, liver and
spleen) are reduced compared to the controls. These results prove
that liposomes are suitable vehicles for a selective drug
delivery in the skin.
Nanoparticles have a
structure similar to liposomes and can therefore perform in a
similar way (Table 2).
Table 2. Performance of
lipid vesicles on the skin
| Nanoparticles |
|
Liposomes |
| |
interact with
stratum corneum enhance skin
humidity
reduce skin
roughness
transport
|
|
| lipophilic
substances |
|
hydrophilic
substances |
|
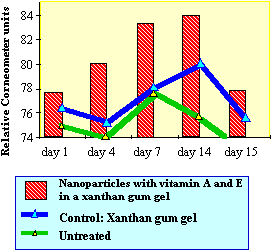 |
| Figure
3. Effect of nanoparticles containing
derivatives of vitamines E and A on skin
humidity determined with Corneometer CM
820. The products (xanthan gum gel 20%
nanoparticles and xanthan gum gel as
control) were applied twice daily on the
forearm of 20 volunteers over a period of
14 days. |
|
|
 |
Figure
3 demonstrates the influence of nanoparticles on skin
humidity. The application of a gel containing
nanoparticles loaded with vitamin A and E derivatives
enhances the skin humidity compared to the controls. The
effect is statistically significant and proves that these
lipid vesicles interact with the stratum corneum. The
increase of skin humidity is due to the high waterbinding
capacity of the phospholipids which form the
nanoparticles. Similar beneficial effects are also
obtained regarding skin roughness by topical application
of nanoparticles (data not shown). However, the main goal
of the treatment is to improve the bioavailability of the
applied vitamins to the skin. It is evident that the
nanoparticles penetrate into the top layers of the
stratum corneum. There they fuse with skin lipids and the
active agents (vitamins) are released. |
The beneficial properties of these vitamin derivatives have been
investigated by in vitro experiments (Figure 4). Mouse fibroblast
cells were cultured in serum free medium containing different
concentrations of nanoparticles loaded with vitamin A palmitate
and vitamin E acetate. A pronounced growth stimulation of these
skin cells could be determined with increasing concentrations of
these lipid vesicles indicating their nutritional and protective
value.
Small
Positively Charged Nanoparticles for Hair Care
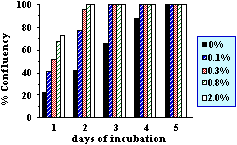 |
| Figure
4. Growth stimulation of mouse
fibroblast cells with nanoparticles in a
serum free medium. The cells were
cultured adherent in tissue flasks at
different concentrations of the
nanoparticles. The nanoparticle
preparation was sterilized by filtration
(0.1µm) and contained 0.6% phospholipids,
0.6% carrier oil, 0.3% vitamine E acetate
and 0.1% vitamine A palmitate. |
|
|
 |
The
formulation of lipophilic substances in hair care
products is unsatisfactory. Conventional oil in water
emulsions used to deliver lipophilic agents to hair and
scalp leave hair feeling sticky and greasy. In addition,
only a poor affinity of the substances to hair is usually
observed. In contrast, nanoparticle preparations with a
vesicle size of less than 50 nm are transparent and do
not feel greasy. These particles represent a new delivery
system to the scalp. The natural phospholipids are well
tolerated emulsifiers which enhance the penetration of
the active agents. Thus the encapsulation of lipophilic
agents in nanoparticles is a very promising galenic
novelty which is easily applicable for the treatment of
disorders like alopecia, dandruff or sunburn. |
| We
have developed a positively charged delivery system to
target encapsulated agents to hair. For that purpose, we
have dotted the shell of nanoparticles with cationic
molecules to get a positive zetapotential [ 11 ]. In our
in vitro experiments, we have encapsulated Uvinul T 150®
(UV-B filter) as active agent. We observed an almost one
hundred fold higher affinity of Uvinul T 150® to hair
from positively charged particles compared to negatively
charged particles (Figure 5). The highly improved
substantivity to hair through positively charged
nanoparticles can also be obtained with other loadings,
such as UV-A sunscreens, vitamins or colors. |
 |
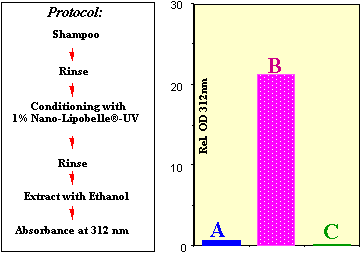 |
| Figure
5. 0.5 g human hair was treated
according to the protocol and incubated
for 5 minutes with 100 ml of two
different preparations (positively and
negatively charged nanoparticles)
containing 6% of UV-B filter Uvinul T-150®.
Bar A shows the amount of UV filter which
could be extracted after treatment with
negatively charged particles. Bar B shows
the value after treatment with positively
charged particles. Control C shows the
value for untreated hair. |
|
|
Conclusions
Phospholipids from soy have successfully been used to prepare
small vesicular carriers for topical application of hydrophilic (liposomes)
and lipophilic (nanoparticles) agents. The technique of high
pressure homogenization permits the industrial production of high
quality vesicle dispersions for cosmetic and dermatological use.
Particle size, surface
charge and payload determine the properties of the preparation
and their application.
Liposomes and
nanoparticles have become an indispensable component of today's
advanced personal care products and have acquired a permanent
place in cosmetic formulations. The phospholipids forming these
carriers enhance the penetration of the active agents into the
stratum corneum and therefore increase their bioavailability. At
the same time, lecithin is also an excellent skin softening and
moisturizing agent itself. Furthermore, sensitive compounds can
be protected with these structures.
The preparation of very
small nanoparticles offers new application possibilities of the
established carrier system. Lipophilic ingredients become water-dispersible
in transparent formulations and have a very high affinity to the
stratum corneum.
Other modifications of
nanoparticles such as the inversion of surface charge, allow the
design of new consumer products for hair care.
Further scientific work in
the field of liposomes and nanoparticles will generate new
properties of these vesicles for commercial applications in
advanced dermatological products.
References:
1 Bangham, A. D., Standish, M. M. and Watkins, J. C.
Diffusion of univalent ions across the lamellae of swollen
phospholipids. J. Mol. Biol. 13, 238-252, 1965
2 Levy, M. Y. and
Benita, S. Design and characterization of submicronized o/w
emulsions of diazepam for parental use. Int. J. Pharm., 54, 103-112,
1989
3 Prankerd, R. J.
and Stella, V. J. The use of oil-in-water emulsions as a vehicle
for parenteral drug administration. J. Parent. Sci. Technol., 44,
139-149, 1990
4 Mayhew, E., Lazo,
R., Vail, W. J., King, J. and Green, A. M. Characterization of
liposomes prepared using a microfluidizer. Biochem. et Biophys.
Acta 775, 169-174, 1984
5 Hoff, E., Nissen,
H. P., Mintel, H. and Kuhs, B. Chemical stabilization of cosmetic
ingredients by means of phospholipid fraction (Probiol). S?FW
120, 530-533, 1994
6 Z?lli, F. and
Suter, F. Preparation of small lipid nanoparticles for topical
applications. Proceed. Intern. Symp. Control. Rel. Bioact. Mater.,
21, 459-460, 1994
7 Egbaria, K. and
Weiner, N. Topical application of liposomal preparations.
Cosmetics & Toileteries 106, 79-93, 1991
8 Junginger, H. E.,
Hofland, H. E J., and Bouwstra, J. A. Liposomes and niosomes.
Cosmetics & Toiletries 106, 45-50, 1991
9 Korting, H. C.,
Zienicke, H., Sch?fer-Korting, M. and Braun-Falco, O. Liposome
encapsulation improves efficacy of betamethasone diproprionate in
atopic eczema but not in psoriasis vulgaris. Eur. J. Clin.
Pharmacol. 39, 349-351, 1990
10 Mezei, M.
Biodisposition of liposome-encapsulated active ingredients
applied on the skin. In O. Braun-Falco, H. C. Korting and H. I.
Maibach, eds, Griesbach Conference on Liposome Dermatics,
Heidelberg: Springer-Verlag, Berlin, 206-214, 1992
11 Z?lli, F.,
Suter, F. and Birman, M. Cationic nanoparticles: A new system for
the delivery of lipophilic UV-filters to hair. Drug &
Cosmetic Industry, 4, 46-48, 1996






